Table of Contents
Polarization is defined by the redistribution of a particle's electronic density due to local electric fields. In the simplest case, the polarizability of two particles with polarizabilities
includes a long range attractive
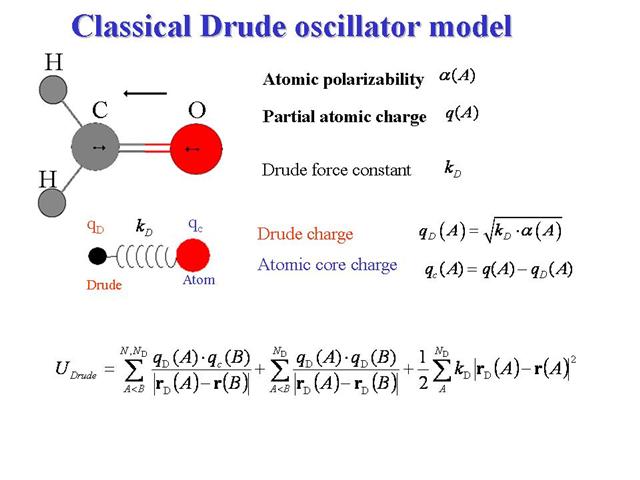
One way to represent polarization in MD is by representing dipoles of finite length as a pair of point charges attached by a harmonic spring, e.g. "shell models" or otherwise referred to as "Drude oscillator models". For the sake of terminology, there is a subtle distinction between shell models, in which dipoles are treated adiabatically, and Drude models, where dipole oscillations are thermal, thereby giving rise to dispersion interaction. The basic schematic is provided below:
There is a positive "core" charge located at the nucleus and a negative "shell" charge with fixed magnitude,
The goal of this program is to determine the potential energy of the induced dipoles, .cif, .pdb, etc.).
The polarization energy is intuitive--it is the energy considering the harmonic spring between the core and shell charges,
where the spring constants
The electrostatic interaction between independent polarizable atoms is written as the sum of the charge-charge interactions between all four charge sites):
Note that the Coulomb interactions between core and shell charges on the same site are typically excluded. Finally, the interaction of the induced dipoles with the static field is written as the sum,
where
Of course, these equations are not without limitations relative to quantum mechanical theory. Namely, polarizable MD models that invoke the shell model depend on approximations of (1) representing the electronic charge density with point charges (or in other methods, dipoles), (2) treating electrostatic polarizabilities isotropically, and (3) terminating the electrostaic interactions after the dipole-dipole term.
Task: For some