-
Notifications
You must be signed in to change notification settings - Fork 0
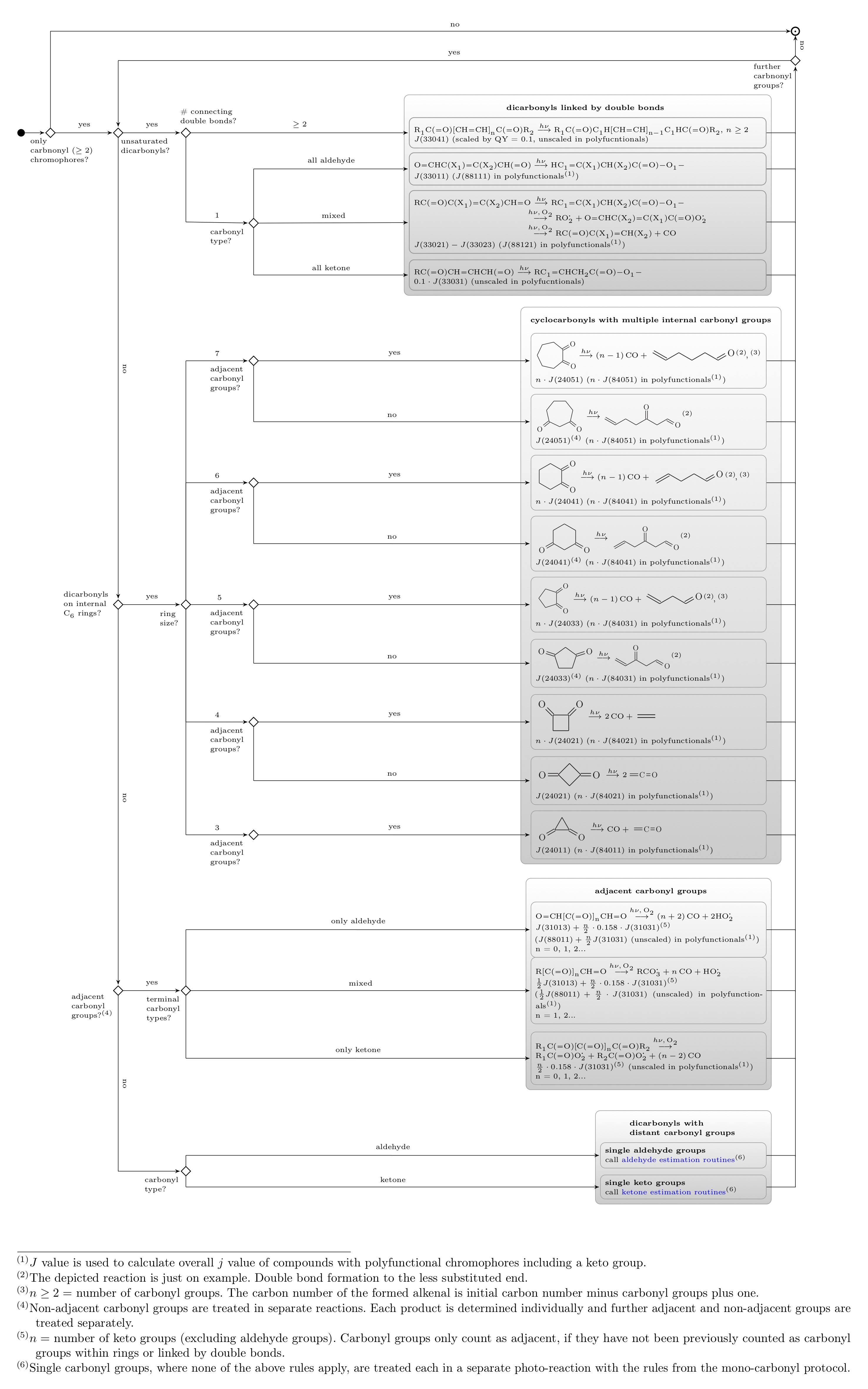
Polycarbonyls
The schematic below details the decision tree for di- and polycarbonyls, i.e. compounds with at least 2 aldehyde and/or keto groups. This includes compounds with additional non-chromophoric substituents such as hydroxyl or carboxyl groups.
The protocol includes rules for α-dicarbonyls and dicarbonyls linked by one or more double-bonded carbon groups. Further rules exist for compounds bearing a C6 ring with internal keto groups or compounds with distant carbonyl groups. For the latter compounds, rules from the mono-carbonyl decision trees are use:
The routine loops over all different carbonyl groups
- α-dicarbonyls
- Dicarbonyls linked by double-bonded carbon groups
- Internal carbonyl groups in C6 ring structures
- Distant single carbonyl groups
and determines the j values and mechanistic data individually. For each carbonyl group, individual photolysis reactions are generated.
Further rules exist for compounds with a carbonyl group and a different chromephore type or for other chromophore types.
Changes in the MCM/GECKO-A version compared to the original TUV
Adding reactions to TUV
Linking TUV to box models
Mono-aldehydes
Mono-ketones
Ketenes
Polycarbonyls
Organic nitrogen compounds
Organic hydroperoxides and PAAs
Criegee intermediates
Polyfunctional chromophores
MCM/GECKO-A reaction numbers
MCMv3.x reaction numbers
MCM/GECKO-A parameterisations