-
Notifications
You must be signed in to change notification settings - Fork 0
Polyfunctional chromophores
In the MCM/GECKO-A photolysis protocol, chromophores are ranked according to their
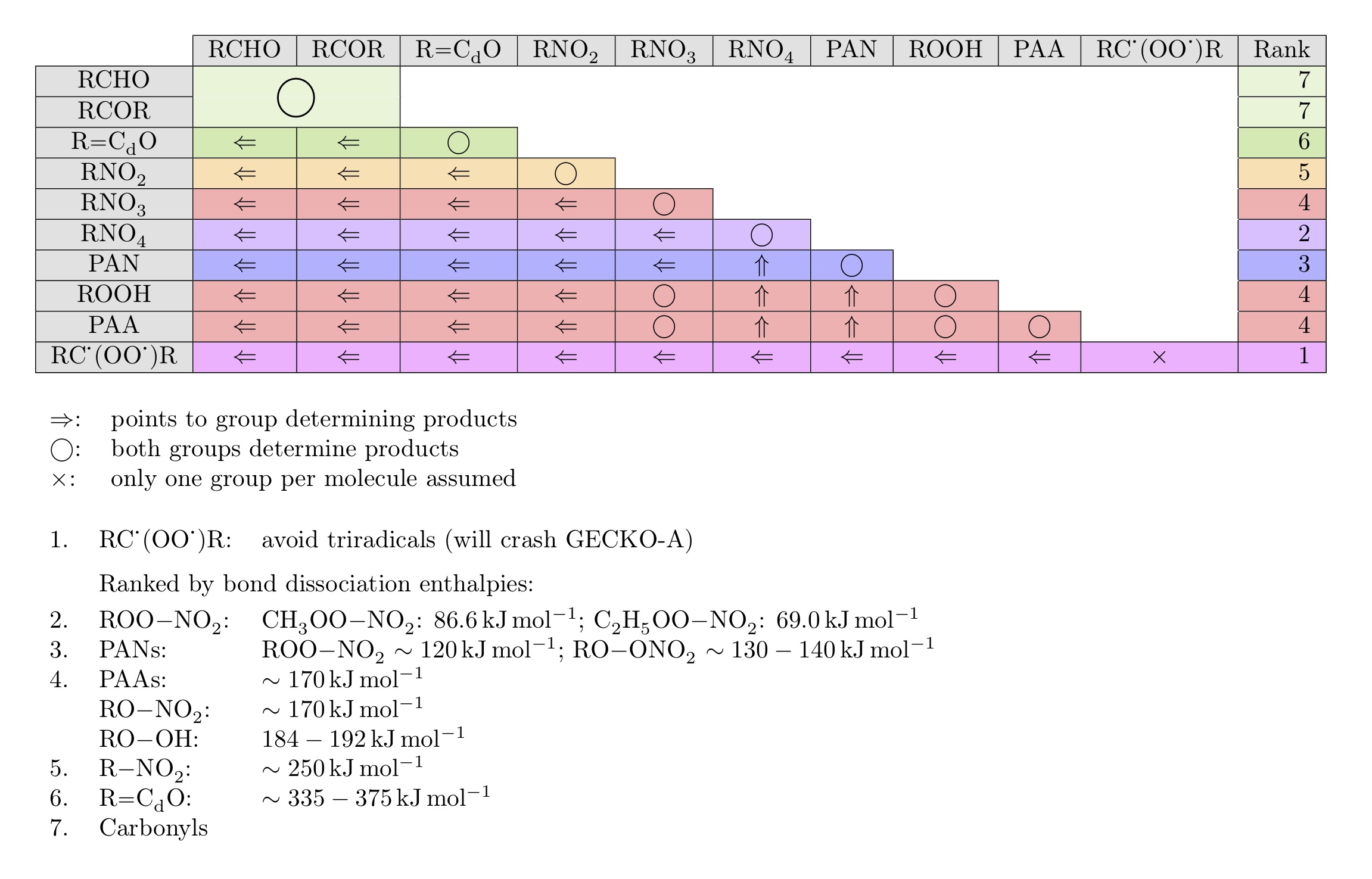
bond strenths (bond dissociation enthalpy or energy, BDE) as shown in the table
below. If a species bears several chromophores of different type, only the chromophore
with the lowest rank determines the products of the photolysis reaction. Criegee
radicals have been given the lowest rank 1 to avoid triradical formation, which
would crash GECKO-A. All other chromophores are ranked by their BDE.
Compounds with multiple chromophores of the same kind follow the rules defined on the protocol pages for the respective chromophore type and don't fall under the rules for compounds with polyfunctional chromophores.
All chromophores contribute with their absorption cross sections to the overall j value, but quantum yields are applied only to the product-determining chromophore with the lowest rank.
This is realised in the generator by summing up individual j values for each chromophore. If quantum yields differ from unity, pressure- and temperature-dependent quantum yields are re-calculated using a quantum yield of 1 for non-product-determining j values. For effective quantum yields, scaling factors are applied to the j values in the photolysis list for single chromophores or multiple chromophores of the same type, and unscaled j values are used in compounds with polyfunctional chromophores.
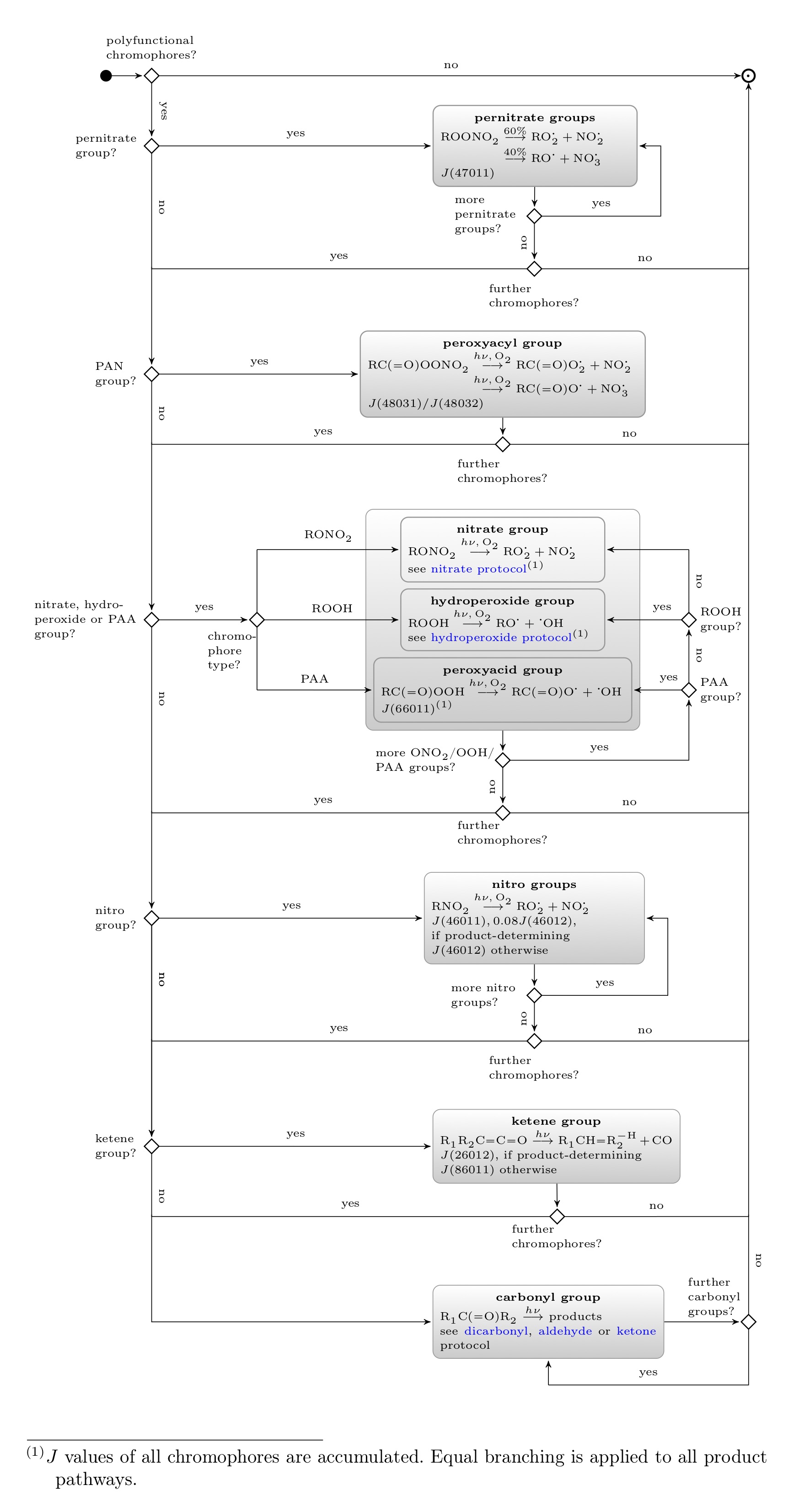
The schematic below details the photolysis of compounds with polyfunctional chromophores. For each chromophore identified, protocol rules of the compound classes with only this chromophore type are applied. All j values are accumulated in an overall j value for this photolysis reaction. Only chromophores in the top box in the schematic below determine the products of the current reaction. All other chromophores only increase the overall j value.
For details of the protocol rules of the various chromophore classes go back to main page and navigate to each class.
Changes in the MCM/GECKO-A version compared to the original TUV
Adding reactions to TUV
Linking TUV to box models
Mono-aldehydes
Mono-ketones
Ketenes
Polycarbonyls
Organic nitrogen compounds
Organic hydroperoxides and PAAs
Criegee intermediates
Polyfunctional chromophores
MCM/GECKO-A reaction numbers
MCMv3.x reaction numbers
MCM/GECKO-A parameterisations