-
Notifications
You must be signed in to change notification settings - Fork 1
How add new item in Adverse Event
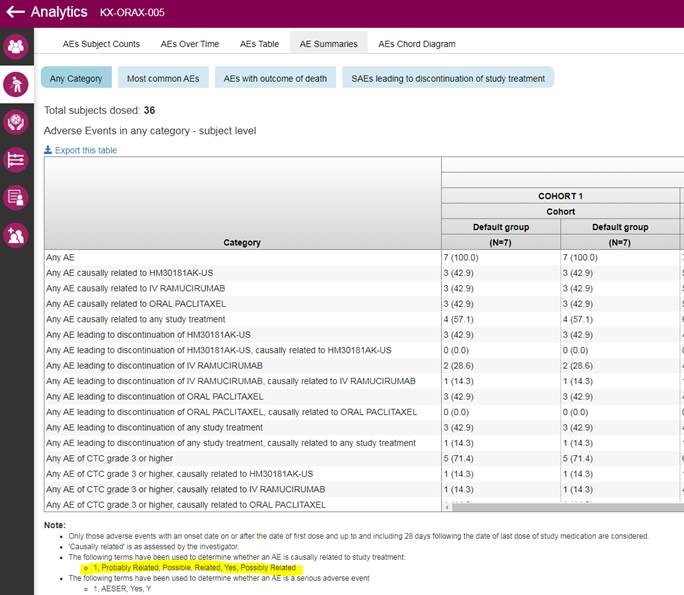
If you need add values ‘Definitely Related’ and ‘Unlikely Related/Not Related’ to the list of terms of “Causality for investigational drugs” in Adverse Event please do following steps:
- First of all you need to upload causality_decode.csv csv file to Azure storage. File content is following:

File content can be changed.
-
In AdminUI open for editing desired dataset and switch to ‘Create mappings to the source data’ screen:
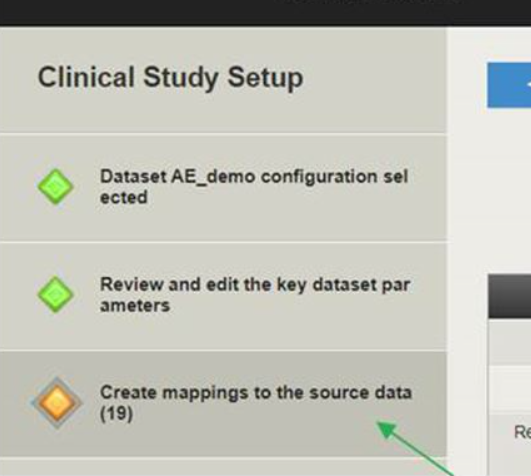
-
Click ‘Add’ button and select option ‘SAS value decoding information’

-
Specify path to decoding file which you previously upload and press Ok:

-
Fields for SAS decoding mapping:

-
Select Adverse Event mapping and for field Causality for investigational drugs in column Decoding Information specify name for decoding column (in attached file it is AECAUS ):

-
Save changes and run ETL .
To use these values decoding mapping should be added to each dataset where you have these values.
- System Requirements
- Azure Setup
- Machine Insights and CBioPortal Integration
- SSL Certificates
- Applications Setup
- Application Spring Configs
- Profiles
- Migrating to ACUITY 9
- Github packages and Docker images
- Result data tables
- Mapping data tables
- Third party solution tables
- Other data tables
- Tables to delete