-
Notifications
You must be signed in to change notification settings - Fork 6
Fluxomics workflow: case studies
By Carles Foguet, Effrosyni Karakitsou, Pedro de Atauri and Marta Cascante (last updated: 2018-08-24)
The following table contains case studies from literature and databases for 13C tracer data based on mass spectrometry, which are applied for estimation of internal fluxes according to the principles of 13C Metabolic Flux Analysis (13C-MFA). Each case corresponds to our free interpretation and adaptation of the data provided by the original authors. The different case-studies are organized around three models of central metabolism:
These models are provided as templates to be used in new analyses. They follow the nomenclature conventions in the BIGG Models data base for genome-scale models: Recon3D for human; iCHOv1 for hamster; iMM904 for Sacharomyces cerevisiae; and iML1515 for Escherichia coli. This permits the integration of 13C-MFA studies with genome-scale models, by adding the results of 13C-MFA as flux constraints to genome-scale metabolic models.
| Reference | Organism/Tissue/Cell line | 13C data | Labelled substrates and products | Suggested escher map | Required input files | |
|---|---|---|---|---|---|---|
| Metabolights MTBLS412 | - | Human HUVEC cells (hypoxia) | Raw data | Glc to Rib, Glycogen, Lac | map | files |
| PMID29922569 | doi | Human MCF-7 breast carcinoma cells (Control) | Corrected data | Gln, Glc to 3PG, Asp, Pyr, Ala | map | files |
| PMID27110360 | doi | Human lung adenocarcinoma A549 cells (21%O2) | Corrected data | Glc, Gln to Mal, N-acetyl-Asp, Cit, AMP, Glu | map | files |
| PMID27379180 | doi | Human pancreatic ductal adenocarcinoma PANC1 cell line (TNF-alpha) | Corrected data | Glc to Pyr, Cit | map | files |
| PMID24773761 | doi | Cricetulus griseus CHO-K1 cell line (Compartimentation) | Corrected data | Glc to Asp, Glu, Gly, Ser | map | files |
| PMID27761435 | doi | Saccharomyces cerevisiae WRY2 (Base strain - glucose) | Corrected data | Glc to Gly, Ala, Val, Thr, Leu, Ile, Asn, Glu, Gln, Arg, Phe, Tyr | map | files |
| PMID16269086 | doi | Saccharomyces cerevisiae (Purely oxidative growth) | Corrected data | Glc to Ala, Gly, Ser, Thr, Asp, Arg, Phe, Tyr, Glu | map | files |
| PMID29142823 | doi | Escherichia coli K12 MG1655 ([0:8:2]) | Corrected data | Glc to Ala, Ser, Thr, Tyr | map | files |
| PMID24957033 | doi | Escherichia coli K12 MG1655 (FAAs) | Corrected data | Glc to Ala, Asp, Glu, Phe, Gly, Val, Tyr, Thr | map | files |
The files required to run each example in the workflow for fluxomics are in the two last columns. There are several possible workflows depending on the selected combination of programs. Among them, two ways of applying the workflow for fluxomics:
- Starting with raw data. This corresponds to the example used in the fluxomics workflow tutorial and includes a complete analysis using Ramid, Midcor, Iso2flux and escher-fluxomics.
- Starting with corrected data obtained from literature. The applications of Ramid and Midcor are not required, and the workflow must be edited to be like this:
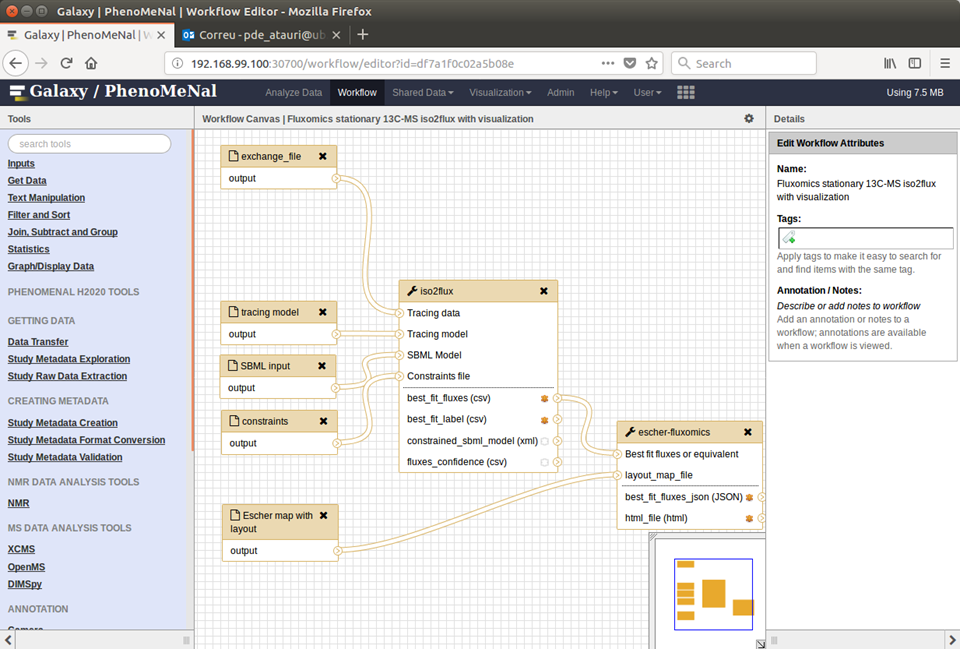
For visualizations of the maps of central metabolism, escher-fluxomics take advantage of the web-based tool Escher and maps available in the community-maps repository for Escher.
Iso2flux is based on a heuristic optimization and, accordingly, complex systems as those presented here might require to run several times the workflow with Iso2flux until a succesful fitting is obtained
Carles Foguet, Effrosyni Karakitsou, Pedro de Atauri and Marta Cascante (Universitat de Barcelona); Pablo Moreno and Namrata Kale (EMBL-EBI). The data covering these case studies were first collected by Molecular Connections and then interpreted and adapted.
 |
Funded by the EC Horizon 2020 programme, grant agreement number 654241 |  |
|---|
